metallic bonds form between
Ionic bonds connect metals to nonmetals and metallic bonds connect a large number of metal atoms. This forms positive metal ions within a sea.
 |
| Metallic Bonding Gidemy Class Notes |
A metallic bond is an impact that holds the metal ions together in the metallic object.
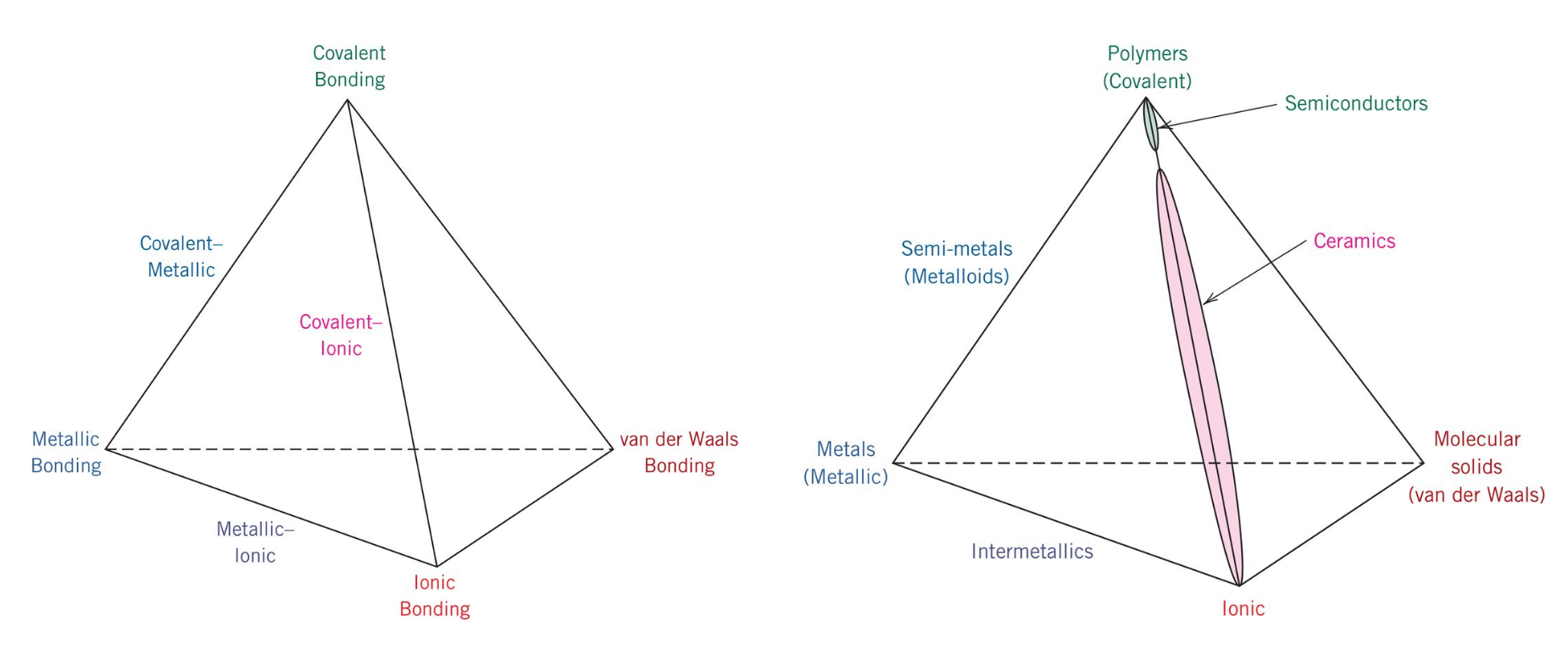
. Ionic bonding is when metal and non-metal share electrons. This bond is observed. Aluminum foil and copper wire are examples of. The electrons within delocalise and are not attached to any particular metal atom.
5 points isabeltirado476 Asked 04232020. Metallic bonds can occur between different elements forming an alloy. What does metallic bonds form between. As far as I understand metals form metallic bonds because they cannot share electrons therefore they almost fuse their outer shells to form giant seas of delocalised electrons.
This bond occurs between metallic elements. Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons and positively charged metal ions. Metallic bonds form between - 15919751 1. It may be described as the.
Metallic bonds are non-directional formed between the delocalized electrons and metal ions. Metals form bonds by merging their outer shell electron orbitals. It is a force of attraction between the metallic cations and the delocalised electrons. Can metallic bonds form between different elements.
What are the characteristics of metallic bonding. It can be as nuclei in a pool of electrons. Mostly in the periodic table left. How do metallic bonds form.
Metallic bonds are formed by valence electrons that interchange freely throughout the metallic crystalline structure. 10 Differences Between Ionic Covalent and Metallic Bonding 1. Metallic bonds are formed only between metal atoms. Metallic bonds are formed within metal atoms.
Covalent bond occurs between the two non-metals metallic bond occurs between two metals and the ionic bond occurs between the metal and the non-metal. Metallic bonds are formed when the charge is spread over a larger distance as compared to the size of single atoms in solids. Covalent bonding occurs between two. These free electrons are responsible for the.
A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cationsIn contrast covalent. Attraction of free floating valence electrons between positively charged metal ions What type of structure do metallic substances have. Metallic bonds form between dissimilar metals in alloys. Metallic bonds are a variation on covalent bonding where the outer orbitals of the metal atoms all overlap allowing the.
 |
| Lesson Explainer Metallic Bonding Nagwa |
:max_bytes(150000):strip_icc()/examples-of-ionic-bonds-and-compounds-603982_Final2-93a47526946144089939cc752ab6cd6a.PNG) |
| Definition And Properties Of Metallic Bonding |
 |
| Bonding Scienceaid |
 |
| Metallic Bond Simple English Wikipedia The Free Encyclopedia |
 |
| Metallic Bonds Video Khan Academy |

Posting Komentar untuk "metallic bonds form between"